In October 2019, the Association of German Engineers (VDI) published a new guideline on the purity of medical devices (VDI 2083 sheet 21). The guideline was developed under the guidance of the Fraunhofer Institute IPA in Stuttgart, which has many years of experience in the area of component cleanliness from the automotive industry. Obviously, this raised awareness of an important topic - in our test laboratory we have seen an increasing demand for cleanliness analysis for medical devices. In this article we inform customers and interested parties about essential aspects of the new directive.
Contamination by particles
In general, the manufacturers of medical devices are responsible for ensuring that their products pose no risk to patients. This particularly includes risks that arise from contamination. In addition to biological and chemical contaminants, which have been routinely investigated for a long time, the consideration of contaminant particles and their risk potential is a more recent aspect, which is addressed in guideline VDI 2083 sheet 21.
Particular requirements regarding particulate contamination have always been placed on liquid medicinal products for injection or infusion into the bloodstream. There is a risk of embolism and vascular occlusion caused by foreign particles. It is therefore logical to place equivalent conditions on the purity of medical devices, provided they come into contact with the bloodstream. Similar considerations apply to medical devices in ophthalmology and for implants.
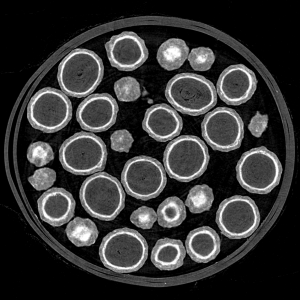
For monitoring particles, the guideline in VDI 2083 Sheet 21 recommends the analytical methods used in the automotive industry for cleanliness analysis which have proven their value and are laid down in international standards (VDA-19 Volume 1 and ISO-16232). The procedure for testing cleanliness consists of the extraction of the particles from the product surface and their filtration onto a membrane. The particle analysis on the membrane is subsequently carried out using microscopic and spectroscopic methods.
Risk-based approach
What is new is the risk-based approach, which enables manufacturers and users to come up with understandable acceptance criteria for the purity assessment, taking existing standards into account. First of all, the basic requirement that medical products must be free of visible particles must be observed. Such dirt particles can trigger allergies or fever, tissue irritation and non-infectious inflammation symptoms in the patient. The requirement can be found in various guidelines and means that there are no particles larger than 100 to 200 µm on the surface of the medical device.
The concrete application of the product must be considered for a further purity assessment. For products with a high risk (eg intravascular catheters, ophthalmic tools) there are also strict requirements for small dirt particles down to 10 µm, which can be used as an acceptance criterion. Impurities due to metallic foreign particles (eg aluminum) occupy a special position, which are increasingly classified as critical due to their toxicity.
Recommendations for manufacturers and users
The new guideline VDI 2083 sheet 21 gives manufacturers and users a tool kit to control the risk for patients from contamination on medical devices. The automotive industry takes a similar approach to ensuring component cleanliness for other reasons. It has been shown here that a one-off certification of products is not enough to maintain quality over the long term. Customers in the automotive industry therefore formulate clear acceptance criteria, which suppliers have to check regularly and independently.
For this reason, we recommend consistent implementation of regular quality controls for medical devices, especially in the critical area of particulate purity, which has not been consistently observed so far. The additional effort for these tests is manageable, but the safety gain for users and patients is considerable.
Do you need advice, testing services or your own laboratory equipment for the implementation of VDI 2083 Sheet 21? Our team of experts will be happy to advise you further.
We look forward to your hearing from you.